Abstract
Background:
Acalabrutinib (ACP-196) is a highly selective, potent, covalent inhibitor of Bruton tyrosine kinase (BTK), in development for the treatment of hematologic malignancies. In the class of irreversible covalent binding kinase inhibitors, the duration of pharmacodynamic activity is a function of the de novo synthesis rate of the target protein, as inhibition of signal transduction is maintained until functional levels of the target kinase are restored (Barf and Kaptein 2012). Acalabrutinib has rapid absorption and a short pharmacokinetic (PK) half-life; twice daily dosing resulted in near complete and continuous BTK inhibition in patients with chronic lymphocytic leukemia (CLL) (Byrd JC et al., 2016).
The objective of this population PK analysis was to describe the PK of acalabrutinib in healthy volunteers (HV) and patients with B-cell malignancies, including mantle cell lymphoma, chronic lymphocytic leukemia, follicular lymphoma, diffuse large B-cell lymphoma, multiple myeloma and Waldenström macroglobulinemia, and investigate effects of covariates on exposure to acalabrutinib.
Methods:
Data originated from 6 Phase 1 trials in healthy volunteers (HV) and 6 Phase 1/2 trials in patients with B-cell malignancies. Rich acalabrutinib concentration data had been collected on Day 1 and Day 8, 14 or 22 (for multiple dose studies). Acalabrutinib concentration-time data were analyzed using a population modelling approach in NONMEM (v. 7.3). Body weight was added to the base model according to allometric principles (Anderson BJ et al., 2008). A full covariate model approach was conducted to investigate if race, sex, health group (Eastern Cooperative Oncology Group [ECOG] performance status [0 vs. 1]), renal (eGFR) and hepatic (NCI Criteria) impairment, dose or concomitant use of acid-reducing agents, resulted in clinically relevant changes in acalabrutinib exposure.
Results:
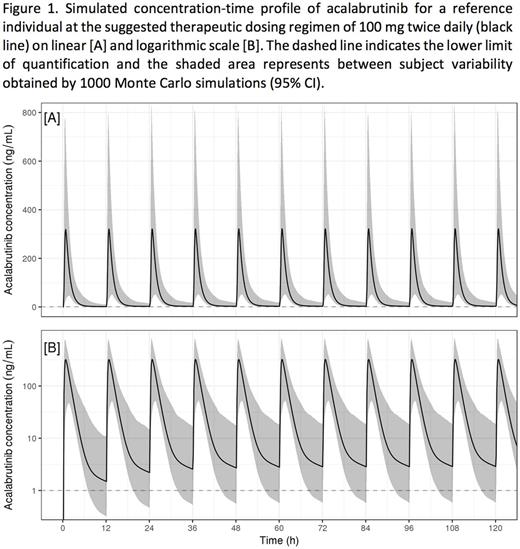
The pooled dataset comprised 11196 acalabrutinib plasma concentrations from 285 HV and 292 patients over a dose range of 15-400 mg. A 2-compartment model with sequential zero- and first-order absorption and a lag time, adequately described the concentration-time profiles. Acalabrutinib had fast absorption and elimination, with no accumulation at the suggested therapeutic dosing regimen of 100 mg BID (fig1). A decrease in apparent clearance (CL/F) was observed with increasing dose. However, the change in CL/F was negligible in the dose range of 75 mg-250 mg and the PK can be considered linear in that range.
Neither age (42 to 90 years), sex, race (Caucasian and African American), ECOG performance status (0 or 1), nor body weight had clinically meaningful effects on the pharmacokinetics of acalabrutinib.
No clinically relevant pharmacokinetic difference was observed in subjects with mild or moderate renal impairment. Acalabrutinib pharmacokinetics have not been studied in patients with severe renal impairment or renal impairment requiring dialysis.
There was no clinically relevant difference in acalabrutinib exposures between subjects with mild or moderate hepatic impairment (NCI Criteria) and subjects with normal hepatic function. Patients with severe hepatic impairment have not been studied.
Use of acid-reducing agents (H2-receptor antagonists and/or proton pump inhibitors) resulted in a 32% decrease in maximum concentration (Cmax,ss) and a 17% decrease in 24h area under the curve (AUC24h,ss). The clinical relevance of this finding is unknown and will be the subject of further investigation.
Conclusions:
The population PK model adequately described the concentration-time profiles of acalabrutinib in healthy subjects and patients.
The suggested therapeutic dose of 100 mg BID did not result in accumulation of acalabrutinib, and in the dose range of 75 mg-250 mg, PK can be considered linear.
The pharmacokinetics of acalabrutinib were not meaningfully affected by age, sex, race, ECOG performance status, mild or moderate hepatic or renal impairment, or body weight. The effect of acid-reducing agents will be further explored.
Edlund: Astra Zeneca: Employment. Andrew: Acerta Pharma: Employment; Astra Zeneca: Equity Ownership. Jin: Theravance Biopharma: Employment. Patel: Acerta Pharma: Employment. Masson: Astra Zeneca: Employment, Equity Ownership. Slatter: Acerta Pharma: Employment; Astra Zeneca: Equity Ownership. Aksenov: Astra Zeneca: Employment. Al-Huniti: Astra Zeneca: Employment.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal